
Science
NH3: What You Need To Know About Its Charge!
Published: January 13, 2024
Learn about the charge of NH3 and its significance in science. Understand the properties and applications of ammonia in this comprehensive guide. Discover everything you need to know about NH3!
(Many of the links in this article redirect to a specific reviewed product. Your purchase of these products through affiliate links helps to generate commission for Noodls.com, at no extra cost. Learn more)
Table of Contents
Introduction
Ammonia, a compound composed of nitrogen and hydrogen atoms, is a vital substance with a wide range of applications across various industries. Its significance in agriculture, industry, and daily life cannot be overstated. Understanding the properties, charge, and uses of ammonia is crucial for comprehending its role in our world.
Ammonia, chemically represented as NH3, is a colorless gas with a pungent odor. It is commonly known for its role in household cleaning products and fertilizers. However, its importance extends far beyond these applications. As we delve into the intricacies of ammonia, we will explore its charge, properties, and diverse uses.
In this article, we will unravel the mysteries of ammonia, shedding light on its charge and delving into the fascinating world of its properties and applications. By the end of this journey, you will gain a comprehensive understanding of ammonia and its significance in various aspects of our lives. Let's embark on this enlightening exploration of NH3!
What is NH3?
Ammonia, with the chemical formula NH3, is a compound made up of one nitrogen atom and three hydrogen atoms. It is a colorless gas with a distinct, pungent odor. This compound is crucial in various industrial processes and has widespread applications in agriculture, household cleaning products, and the manufacturing of numerous everyday items.
In nature, ammonia is produced through the decomposition of organic matter, including animal waste and plant materials. It can also be synthesized through the Haber process, a method that combines nitrogen and hydrogen under high pressure and temperature to produce ammonia. This synthetic ammonia plays a pivotal role in the production of fertilizers, contributing significantly to global agricultural practices.
Ammonia is highly soluble in water, forming a strongly alkaline solution. This property makes it a versatile compound in various chemical processes and applications. Its ability to form ammonium compounds, such as ammonium nitrate and ammonium sulfate, further expands its utility in agricultural fertilizers and soil treatments.
In addition to its role in agriculture, ammonia is a key component in the manufacturing of cleaning products. Its alkaline properties make it an effective cleaner for surfaces, floors, and even certain fabrics. Furthermore, ammonia is utilized in the production of various synthetic materials, including plastics, fibers, and pharmaceuticals, highlighting its significance in industrial processes.
It is important to note that while ammonia possesses numerous beneficial properties, it also poses health and safety risks. Exposure to high concentrations of ammonia can cause irritation to the respiratory system and eyes, emphasizing the importance of handling this compound with care and caution.
In summary, ammonia, with its distinct odor and versatile properties, plays a multifaceted role in agriculture, industry, and everyday life. Its significance extends from the production of fertilizers to the manufacturing of cleaning products and synthetic materials. Understanding the nature of ammonia is essential for appreciating its impact on diverse aspects of our lives.
Charge of NH3
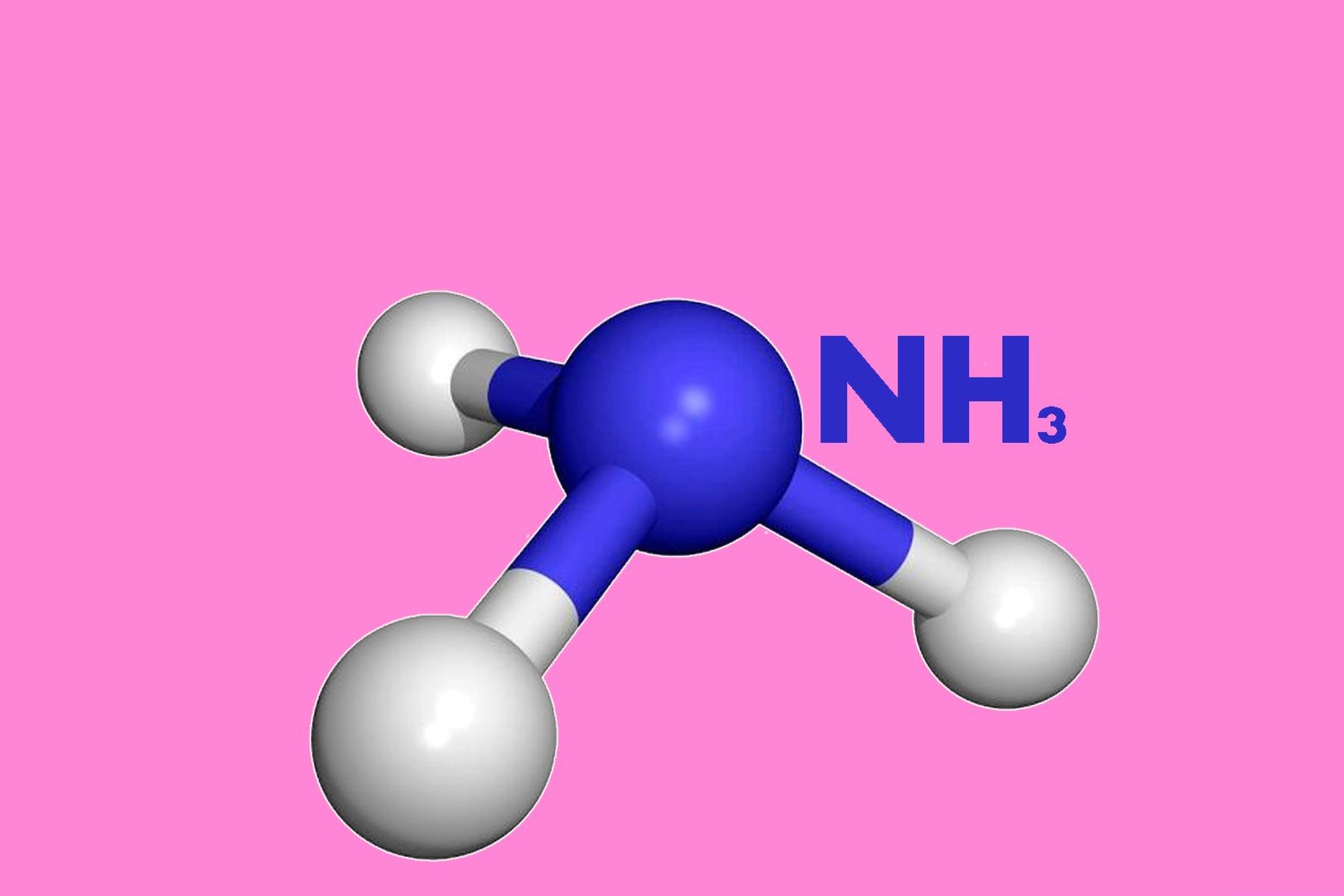
Ammonia, with the chemical formula NH3, possesses a unique charge that contributes to its diverse properties and reactivity. The charge of NH3 is a result of its molecular structure and the distribution of electrons within the compound.
In NH3, the nitrogen atom is covalently bonded to three hydrogen atoms. This arrangement gives the nitrogen atom a lone pair of electrons, which contributes to the compound's overall charge. The lone pair of electrons on the nitrogen atom gives ammonia its characteristic basic properties. This means that ammonia can act as a Brønsted-Lowry base, readily accepting protons in chemical reactions.
The lone pair of electrons on the nitrogen atom is responsible for the compound's ability to form bonds with other molecules, particularly with substances that contain acidic hydrogen atoms. This reactivity is crucial in various chemical processes and biological reactions, making ammonia a key player in numerous industrial applications and biological systems.
The charge distribution within the ammonia molecule also influences its solubility and interactions with other compounds. Due to the presence of the lone pair of electrons, ammonia readily forms hydrogen bonds with water molecules, leading to its high solubility in water. This property is essential in the use of ammonia-based solutions in cleaning products and industrial processes.
Furthermore, the charge of NH3 contributes to its role as a crucial component in the formation of ammonium compounds. When ammonia reacts with acids, it forms ammonium salts, such as ammonium nitrate and ammonium sulfate. These compounds have widespread applications in agriculture as fertilizers, providing essential nutrients to plants and enhancing crop yields.
Understanding the charge of NH3 is fundamental in comprehending its behavior in various chemical reactions and applications. The lone pair of electrons on the nitrogen atom and its ability to form hydrogen bonds contribute to the compound's reactivity, solubility, and role in the formation of important agricultural fertilizers. This insight into the charge of NH3 provides a deeper appreciation of its significance in the realms of chemistry, agriculture, and industry.
Properties of NH3
Ammonia, with the chemical formula NH3, exhibits a fascinating array of properties that contribute to its diverse applications and significance in various fields. Understanding these properties is essential for comprehending the compound's behavior in different environments and chemical reactions.
1. Pungent Odor: One of the most distinctive properties of ammonia is its pungent odor. Even at low concentrations, ammonia's smell is easily detectable, often described as sharp and unpleasant. This characteristic odor serves as a warning sign of ammonia's presence, alerting individuals to its potential health risks.
2. Solubility in Water: Ammonia is highly soluble in water, forming an alkaline solution. This property is crucial in the production of ammonia-based cleaning products and the formulation of aqueous ammonia solutions used in various industrial processes. The solubility of ammonia in water also facilitates its role in agricultural practices, where it is utilized in the form of ammonium-based fertilizers.
3. Basicity: NH3 exhibits basic properties due to the lone pair of electrons on the nitrogen atom. This allows ammonia to act as a Brønsted-Lowry base, readily accepting protons in chemical reactions. Its basic nature makes it a versatile compound in various chemical processes and biological systems.
4. Reactivity: Ammonia demonstrates reactivity with a wide range of substances, particularly with compounds containing acidic hydrogen atoms. This reactivity is fundamental in the formation of ammonium salts, which are essential components of fertilizers and play a pivotal role in enhancing soil fertility and promoting plant growth.
5. Flammability: While ammonia itself is not flammable, it can support combustion in the presence of certain substances. This property necessitates caution in handling and storing ammonia to prevent potential fire hazards.
6. Toxicity: Exposure to high concentrations of ammonia can be harmful to human health, causing irritation to the respiratory system and eyes. Understanding the toxic properties of ammonia is crucial for implementing safety measures in industrial settings and ensuring the safe use of ammonia-based products.
7. Versatility in Industrial Processes: The unique properties of ammonia make it an indispensable compound in various industrial processes, including the manufacturing of synthetic materials, refrigeration systems, and the production of nitrogen-based chemicals.
In summary, the properties of ammonia, ranging from its pungent odor and solubility in water to its reactivity and basic nature, underpin its multifaceted role in agriculture, industry, and everyday life. These properties shape the compound's behavior and reactivity, highlighting the diverse applications and significance of NH3 in numerous domains.
Uses of NH3
Ammonia, with its versatile properties and reactivity, finds widespread applications across various industries and everyday activities. The diverse uses of NH3 underscore its significance in agricultural practices, industrial processes, and household applications.
-
Agricultural Fertilizers: One of the primary uses of ammonia is in the production of nitrogen-based fertilizers. Ammonia serves as a key component in the synthesis of ammonium nitrate and ammonium sulfate, which are essential for enriching soil fertility and promoting plant growth. These fertilizers play a crucial role in modern agriculture, contributing to increased crop yields and sustainable farming practices.
-
Cleaning Products: Ammonia-based cleaning products are widely utilized for their effectiveness in removing dirt, grease, and grime from various surfaces. The alkaline nature of ammonia makes it an excellent degreaser and cleaner for floors, glass, and household appliances. Its ability to dissolve in water enables the formulation of cleaning solutions that are used in households, commercial establishments, and industrial settings.
-
Refrigeration Systems: In the realm of industrial refrigeration, ammonia is valued for its thermodynamic properties and environmental sustainability. It is commonly used as a refrigerant in large-scale industrial refrigeration systems, providing efficient cooling while minimizing environmental impact. The use of ammonia as a refrigerant aligns with the global efforts to transition towards eco-friendly and energy-efficient cooling technologies.
-
Synthetic Materials Production: Ammonia serves as a precursor in the production of various synthetic materials, including plastics, fibers, and pharmaceuticals. Its role in the manufacturing of synthetic materials highlights its significance in the chemical industry, where it contributes to the production of a wide range of essential products that are integral to modern life.
-
Wastewater Treatment: In the realm of environmental engineering, ammonia plays a vital role in wastewater treatment processes. It is utilized in the form of aqueous ammonia solutions to adjust pH levels and facilitate the removal of contaminants from wastewater. The use of ammonia in wastewater treatment underscores its importance in environmental remediation and sustainable water management practices.
-
pH Control in Industrial Processes: Ammonia's alkaline properties make it valuable for pH control in various industrial processes. It is employed to adjust the acidity or basicity of solutions, contributing to the optimization of chemical reactions and industrial operations across diverse sectors.
-
Textile Industry: Ammonia is utilized in the textile industry for processes such as pre-treatment, dyeing, and printing of fabrics. Its role in the textile industry highlights its significance in facilitating essential manufacturing processes and the production of a wide range of textile products.
The diverse uses of NH3 underscore its indispensable role in agriculture, industry, and environmental engineering. From enhancing soil fertility to serving as a key component in cleaning products and industrial processes, ammonia's versatility and reactivity make it an essential compound that contributes to numerous facets of modern life.
Safety Precautions with NH3
When handling ammonia (NH3), it is crucial to adhere to stringent safety precautions to mitigate potential health risks and ensure safe usage in various applications. The pungent odor and reactivity of ammonia necessitate careful handling and storage practices to minimize exposure and prevent accidents.
Personal Protective Equipment (PPE)
Individuals working with ammonia should wear appropriate personal protective equipment, including goggles, gloves, and respiratory protection. This safeguards against potential eye and skin irritation, as well as respiratory issues resulting from ammonia exposure.
Ventilation
Ammonia should only be used in well-ventilated areas to prevent the accumulation of high concentrations of the gas. Adequate ventilation helps disperse ammonia vapors, reducing the risk of inhalation and ensuring a safe working environment.
Storage and Handling
Proper storage and handling of ammonia are essential to prevent leaks and spills. Ammonia should be stored in tightly sealed containers in well-ventilated areas, away from incompatible substances. Additionally, it is crucial to follow strict protocols for transferring and transporting ammonia to minimize the risk of accidents and exposure.
Emergency Response Preparedness
Facilities working with ammonia must have comprehensive emergency response plans in place. This includes training personnel on proper response procedures in the event of ammonia leaks, spills, or exposure incidents. Having appropriate safety equipment and emergency response resources readily available is critical for swift and effective mitigation of potential ammonia-related incidents.
Regulatory Compliance
Adherence to relevant safety regulations and guidelines is paramount when working with ammonia. Facilities must comply with occupational safety standards and environmental regulations to ensure the safe handling, storage, and usage of ammonia while minimizing environmental impact and protecting the well-being of workers.
Risk Assessment and Training
Conducting thorough risk assessments and providing comprehensive training to personnel are essential components of ensuring safe practices with ammonia. Educating workers about the potential hazards of ammonia, safe handling procedures, and emergency response protocols is crucial for mitigating risks and fostering a culture of safety within the workplace.
Monitoring and Maintenance
Regular monitoring of ammonia storage and usage areas, as well as proactive maintenance of equipment and storage facilities, is vital for early detection of potential issues and the prevention of leaks or releases. Implementing routine inspections and maintenance protocols helps uphold safe working conditions and prevent hazardous situations.
By prioritizing these safety precautions and practices, the risks associated with handling ammonia can be effectively managed, ensuring the well-being of personnel and the safe utilization of ammonia in various industrial, agricultural, and household applications.